Code with Care: The Ins and Outs of Medical Device Software

When people think about medical devices, most might picture big, complicated equipment that seems too cumbersome for use outside hospital settings. Progress, however, does not stand still. Advances in technology and electronics have made health monitoring devices accessible and usable anywhere, thanks to medical device software (MDS). This type of software can replace some traditional healthcare equipment with smartwatches and smartphones we all use daily and has as much potential to improve our health outcomes.
As a healthcare software development service provider, we’ve created and tested software for diverse healthcare-related projects. From our experience, there are many intricacies of medical device software development, and we’re here to guide you through them. We’ll touch upon regulations, review different types of medical software, and share the steps our team uses to develop digital health solutions.
from 25 countries outsourced software development to Relevant
We provide companies with senior tech talent and product development expertise to build world-class software.
Medical Device Software: Overview
The story of medical device software started with simple goals: make hospitals work smoother and patient care better. Early on, this meant software that helped keep track of all the patient info or made that big, complex equipment a bit smarter. From the early days when software was simply firmware or embedded systems within medical devices (SiMD), its capabilities have been growing alongside processors and electronics that became smaller yet more powerful and affordable.
Back in the ’90s, when the Internet and laptops started getting big, this set the stage for significant technological advancements. Followed by smartphones in the 2000s and then cloud computing, AI, and the Internet of Things in the 2010s, these developments profoundly changed the game for the software we use today. Modern healthcare digital solutions are now highly advanced and connected to databases and remote systems. This newer generation of software has evolved from being a component of medical processes to being at the heart of healthcare progress.

The figures say the same. Statista predicts that the value of the medical device software market will jump from its present $511 billion to an impressive $719 billion by the year 2028.
Reasons Why Companies Need Software for Medical Devices
Although the potential revenue and increasing market value are a big attraction, they’re not the sole reasons organizations are investing in custom medical device software engineering.
In essence, the development of MedTech software brings a host of advantages for healthcare entities:
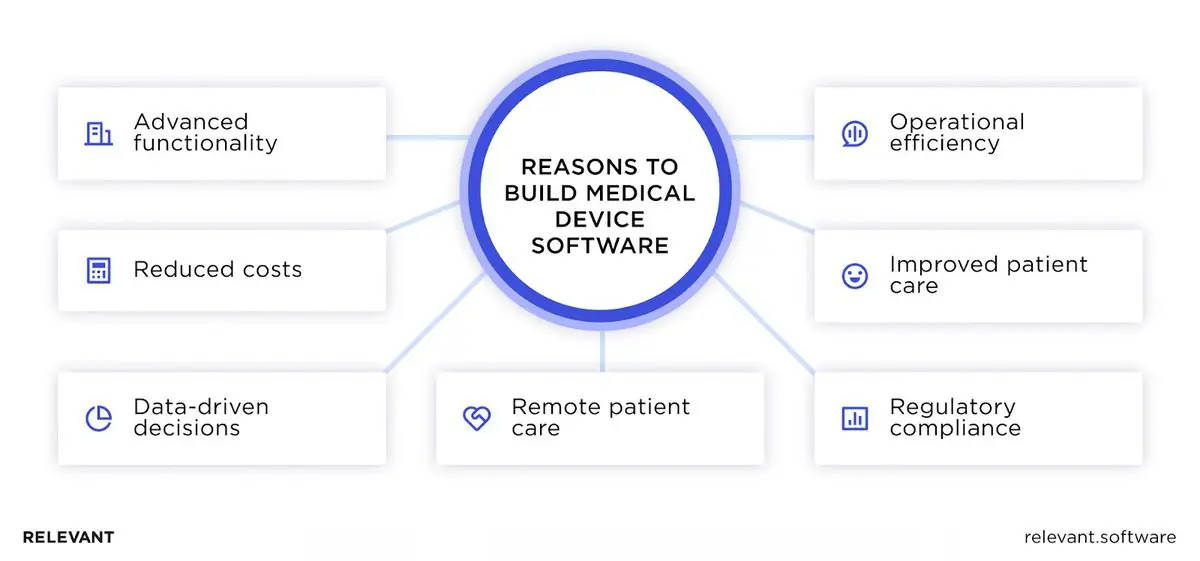
- Better care for patients. Custom software for medical instruments lets doctors watch over patients in real time, diagnose accurately, and come up with treatments that work, leading to happier, healthier patients.
- Efficiency in operations. Medical device software takes the boring tasks off medical staff’s plates, cuts down on all that paper, and helps everything flow better. So healthcare teams can spend more time doing what they do best—caring for patients.
- Remote patient monitoring. With this tech, caring for patients doesn’t have to mean their necessary presence in hospitals. MDS facilitates telehealth services and lets healthcare specialists offer quality care over the Internet and reap the benefits of remote patient monitoring.
- Regulatory compliance. When it comes to medical software, you’ve got to play by some pretty strict rules, or you’ll face some serious fines. Custom-built software can be tailored to these specific standards, which ensures device safety for patients and keeps the financial side in check.
- Innovative features. Thanks to technologies like AI and machine learning, we can do things like predict health issues before they happen and tailor care to each person’s needs, moving past old-school methods.
- Lower operational costs. When software takes over some of the routine work and makes devices do more, hospitals end up saving money. As a result, everyone can get top-notch healthcare without breaking the bank.
- Data-driven decisions. Software-equipped medical devices generate vast amounts of real-world data, which can be analyzed to inform better healthcare decisions. This way of using data helps spot health trends, guess what might happen next, and tailor care just for you, making sure everyone gets the treatment that fits them best.
As you can see, developing healthcare apps and software for medical needs is a sound investment. But, creating medical software demands specialized expertise and a solid grasp of healthcare regulations. That’s why a lot of health organizations team up with experienced custom software development companies who know the ins and outs and can guarantee the success of the project.
But before we examine the compliance rules more closely, let’s understand the different kinds of medical software solutions.
Types of Medical Device Software
Complete clarity around how the medical device software you’re developing or planning to develop qualifies is critical, as it will tell you what industry regulations your software must meet. Therefore, let’s understand the two main types of it:
| SiMD | SaMD | |
| Purpose | Directly manages device functions, processes data, and delivers therapy as part of the device’s intended use. | Doesn’t directly control device functions, but analyzes data, provides insights, or facilitates communication between devices. |
| Device Integration | Embedded within a device | Standalone software, but can interact with and utilize data from medical devices. |
| Device Association | Designed and validated for a specific device or a compatible set. | Can work with a specific device or group of compatible devices depending on functionalities. |
| Device Dependence | Heavily dependent on the specific hardware and functionalities of the associated device. | Operates independently of specific hardware but may require data from or interact with medical devices. |
| Regulation | Typically falls under higher risk classifications due to its direct impact on patient safety and device operations. | Can fall under lower-risk classifications depending on functionalities and potential impact, but still subject to regulations. |
| Examples | Infusion pump software for calculating and administering insulin doses, image processing software that processes medical images displayed by the device. | Mobile app providing personalized exercise recommendations based on wearables data, software analyzing medical images from a diagnostic device. |
| Key Differences | Embedded, directly controls device functions, higher risk classification. | Standalone, supports and enhances device function, potentially lower risk classification. |
Software in Medical Devices (SiMD)
SiMD or Software in a Medical Device is essentially software embedded within a physical medical device, responsible for its operation and functionalities. Putting simply, any software that operates a physical medical device, whether it’s driving the device’s mechanics or creating its user interface, falls under the category of SiMD. This kind of software is often referred to as “firmware,” “embedded software,” or “micro-code.” Some examples of SiMD include:
- Software that monitors heart rhythm, calculates appropriate responses and delivers electrical impulses in pacemakers and defibrillators
- Software that processes and analyzes magnetic resonance images in MRI devices
- Software that controls the delivery of medication based on pre-programmed settings and patient data in infusion pumps
Standalone Software as a Medical Device (SaMD)
SaMD, or Software as a Medical Device, is software that operates independently of any hardware medical device. This type of software is capable of running on general-purpose computing platforms (such as smartphones, tablets, or laptops) and does not control or interact directly with the mechanics of a hardware device. While it can carry one or more medical functions (diagnosing, health monitoring, or treatment recommendations), it shouldn’t be a part of hardware to be categorized as software as medical device. As strange as it may sound, it’s considered a medical device, and here are a few of such medical device examples:
- Mobile applications that analyze images for skin lesion diagnosis and use deep learning algorithms to identify potential skin cancers.
- Software platforms that intake patient health records to predict disease risk or customize treatment plans.
- Mental health apps that monitor user input and activity to provide cognitive behavioral therapy or stress management techniques.
- Diagnostic algorithms that interpret data from wearable devices, such as smartwatches, to detect arrhythmias or other cardiac conditions from an electrocardiogram (ECG) reading.

So, regardless of specific role, whether it’s
- Patient-specific software
- Diagnostic software
- Medical device tracking software
- Therapeutic software
- Clinical decision support software
they can fall under SiMD or software as a medical device, depending on their implementation and functionality. Some software for clinical decision support could combine the elements of both. For example, an app displaying data from a wearable device while providing analysis could be considered a combination of SiMD (interacting with the device) and SaMD (offering independent analysis).
Ultimately, the classification hinges on a specific regulatory assessment considering:
- Interaction with physical devices: How directly does the software interact with and control these devices?
- Impact on patient care: Does it directly influence treatment decisions or patient safety based on the tracked information?
- Regulatory context: Depending on the jurisdiction and specific functionalities, the classification might vary.
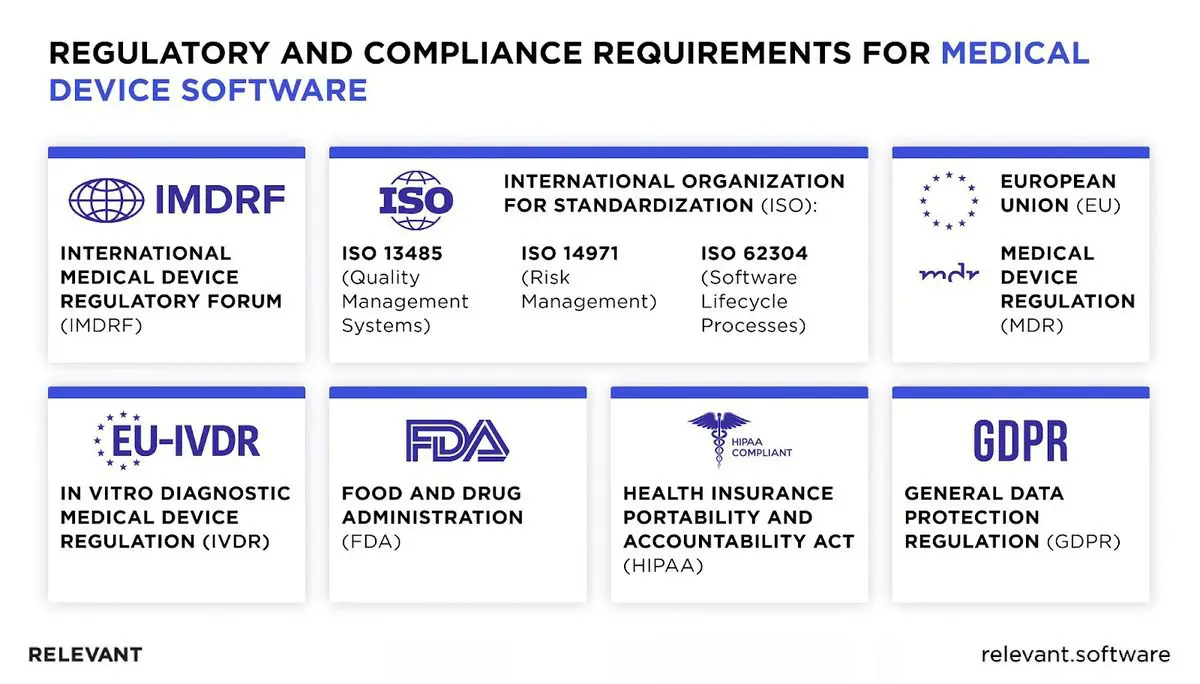
Regulatory Landscape for Medical Device Software
With various international and regional medical device software standards in place, understanding these regulations is essential to ensure medical tools’ quality, safety, and effectiveness. The EU and US markets for medical devices are the largest by far, so we’ll focus on them.
- FDA Guidelines and Approval Process
Since SiMD and software as medical device are primarily viewed as medical devices, they are regulated as such. The FDA sorts medical devices into three risk-based classes, with Class I being the lowest risk and Class III the highest, determining the regulatory controls and approval path needed.
| State of healthcare situation or condition | Treat or diagnose | Drive clinical management | Inform clinical management |
| Critical | IV | III | II |
| Serious | III | II | I |
| Non-serious | II | I | I |
For most Class II and some Class I devices, a 510(k) submission is necessary, proving your device matches another on the market. Class III devices require Pre-market Approval (PMA), showing through scientific evidence that the device is safe and effective. All devices must comply with the Quality System Regulation (QSR), which sets standards for manufacturing, quality control, and documentation, regardless of the device’s class.
Here are compliance steps for you to follow:
- Determine your device’s class. Start by identifying which of the FDA’s classes your software-only device falls into.
- Prepare your submission. Depending on your device’s classification, prepare the necessary 510(k) submission or PMA application (detailed information about your device, its intended use, and evidence of its safety and effectiveness).
- Implement quality systems. Establish and maintain a quality system that complies with the FDA’s QSR. This should be in place before you submit your application and continue throughout the life of the device.
- Submit to the FDA. Once your submission is ready and your quality systems are in place, submit your application to the FDA and await their review.
- FDA Review and Approval: The FDA will review your submission to ensure it meets all regulatory requirements. This process can take from a few months to a few years, depending on the device’s complexity and risk.
- Post-Market Surveillance: After approval, you must comply with post-market surveillance requirements, which include reporting any adverse effects and conducting regular audits of your quality system.
- Global Regulatory Variations
Unlike the FDA’s approach in the United States, which classifies devices based on risk and has distinct pathways like 510(k) and PMA for device approval, the European Union’s Medical Device Regulation (EU MDR) emphasizes clinical evidence, post-market surveillance, and a unique device identification system to enhance traceability. Other international standards, such as the International Organization for Standardization (ISO) series for medical devices (like ISO 13485 for quality management systems), play a crucial role in harmonizing global regulatory requirements.
- Cybersecurity and Data Privacy in Medical Software
In the US, the Health Insurance Portability and Accountability Act (HIPAA) is a critical aspect of medical device software because it sets the standard for protecting sensitive patient data. It requires healthcare providers and their business associates to safeguard electronic protected health information (ePHI) with both physical and technical measures.
Europe has a similar standard, the General Data Protection Regulation (GDPR), which imposes strict rules on data privacy and security and demands the implementation of appropriate security measures. Compliance with these rules and regional laws has one goal – to encourage medical software developers to embed robust security features (encryption, access controls, and regular security audits) from the ground up.
Development and Testing of Medical Software
The medical device software development requires strong engineering expertise and goes through the same stages as any other type of software, with the addition of a more complicated validation stage and regulatory approval. Here are the main steps and important aspects you should consider when building your medical software product.
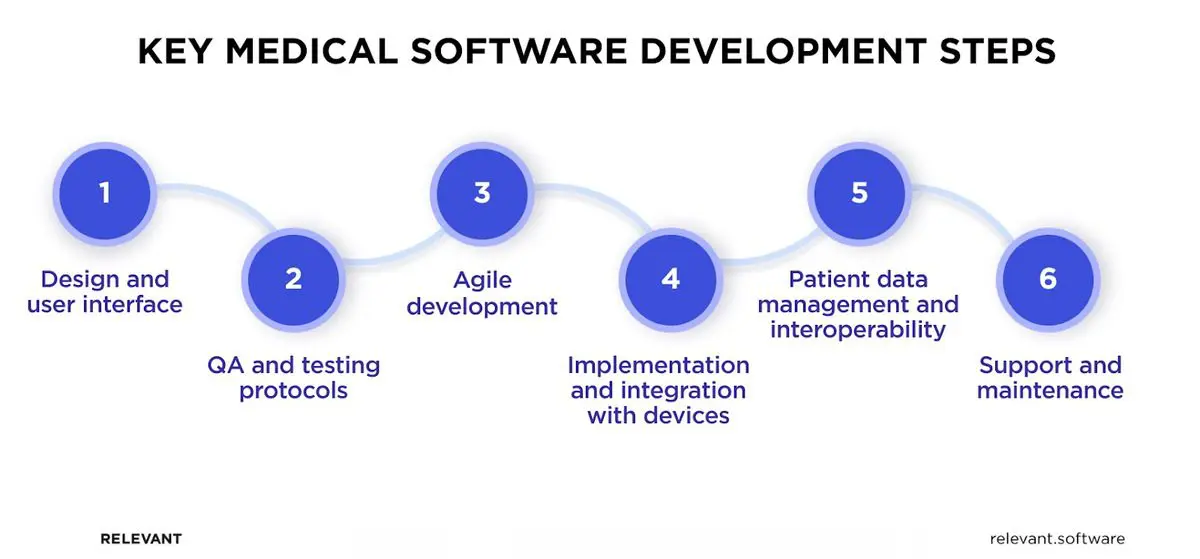
Design Considerations and User Interface
Today, users’ expectations are so high that user-friendly interfaces and visual appeal are a must. Otherwise, they will just leave. That’s why the design has to be clear and straightforward, whether it’s for patients’ use or doctors’ who are working under a lot of pressure.
The interface should be comfortable for a tech-savvy young adult yet simple enough for older individuals who might not be as familiar with technology. Accessibility is another critical aspect of medical device software design, so don’t neglect it. Also, remember, the main goal of a good design is to help users quickly get to the most important functions and info they need.
Quality Assurance and Testing Protocols
Medical coding software deals with lives, not just likes. A malfunction or bug can have catastrophic consequences. QA and testing services ensure that your software meets stringent regulatory requirements and functions as intended, mitigating risks and building trust with patients and healthcare professionals.
Here are key QA and medical device software testing protocols you should pay attention to:
- Risk management. Identifying and assessing potential risks throughout the development lifecycle is paramount. You can employ tools like Failure Mode and Effects Analysis (FMEA) and Hazard Analysis for this purpose.
- Requirements management. Clearly defined and documented requirements ensure the software aligns with its intended purpose and regulatory standards. Traceability between requirements, design, and testing is key.
- Software testing. Rigorous testing across various levels (unit, integration, system, acceptance) verifies the software functions as expected and complies with requirements.
- Verification and validation. Verification confirms the software is built correctly, while medical device software validation ensures it meets user needs and performs as intended in real-world scenarios.
Agile Development in Medical Software Development
How to embrace the agility and speed of modern development methodologies while ensuring the rigorous quality and compliance required for medical software? Even though regulations are laid out in a sequential manner that aligns well with a traditional waterfall development method, it’s very much possible to employ an agile approach while still adhering to all necessary standards.
By making some adaptations to the agile software development life cycle, you can make it work for your medical software:
- Agile-compliant processes. Using frameworks like DSDM or SAFe will help you adhere to strict standards like IEC 62304 while using an agile development methodology for medical software.
- Early and continuous testing. Integrate automated testing tools and build quality into each sprint.
- Version control. Manage documentation changes and traceability thoroughly.
Implementation and Integration with Smart Devices
At this stage, you should integrate your software with hardware devices (smartphones, smartwatches, or specific medical equipment) and ensure smooth interaction with them. There are several ways to facilitate the medical device integration software process.
First, you can connect your software using the APIs provided by smart devices and medical equipment makers. Another way is cloud-based platforms, which can simplify the storage, analysis, and sharing of data across various devices. For scenarios where off-the-shelf solutions don’t quite fit, custom development is a sure way to go.
Patient Data Management and Interoperability
Medical devices generate a wealth of valuable patient data, but managing it effectively poses several challenges:
- Standardization. Diverse formats and communication protocols across devices create silos of information, hindering seamless exchange and analysis.
- Privacy. Balancing data accessibility for healthcare professionals with patient privacy rights requires careful consideration.
- Integration. Integrating data from multiple devices with existing healthcare IT infrastructure can be complex and time-consuming.
Interoperability, the ability of devices and systems to exchange data seamlessly, holds the key to overcoming these challenges. Here are the best ways to achieve that interoperability:
- Adhere to established FHIR standard to facilitate communication and interoperability.
- Partner with HL7 FHIR implementation service providers, technology companies, and regulatory bodies to develop and adopt interoperable solutions.
- Design user-friendly interfaces and workflows for both patients and healthcare professionals interacting with the data.
Support and Maintenance
While developing software is the main part of the product life cycle, its journey doesn’t end there. Ongoing support, regular updates, and functionality improvements are continuous processes you should invest in if you want to keep your medical device software secure and compliant.
Trends and Innovations in Medical Device Software
With clinical advances and tech innovations happening on all fronts of the healthcare industry, here are some of the key medical software trends you should keep an eye on in 2024:
- AI and Machine Learning Integration
More and more medical software products leverage artificial intelligence due to the many benefits of AI in healthcare. This technology contributes to the software’s capabilities to diagnose more accurately, personalize treatment plans, and monitor conditions on a whole new level.
- Telemedicine and Remote Monitoring Software
In fact, this trend gave rise to the software as a medical device adoption by empowering software to collect patient vital statistics remotely. No doubt, telemedicine solutions will continue to dominate the market and keep the demand for medical software in the foreseeable future.
- Wearable Technology and Mobile Health
Considering the huge popularity of wearables and mobile health apps amongst fitness chronic patients, enthusiasts, and the elderly, these are trending tech medical solutions used in software right now. They offer an endless array of functions aimed at patient care, which are expanding at a constant rate.
Build Medical Device Software with Relevant Software
Dealing with strict regulations and constantly shifting trends when developing medical device software may seem insurmountable, yet with the right approach and people, entirely manageable.
If you have Relevant Software on your side, your organization can surely build high-quality healthcare software with ease. Our team, experienced in the nuances of medical software, will share its expertise to help you overcome all the complexities of healthcare software design. Don’t let the challenges hold you back. Contact us, and let’s create healthcare solutions that make a difference.


Hand-selected developers to fit your needs at scale! Let’s build a first-class custom product together.

