How AI in Pharma Cuts Costs and Speeds Discovery: Pharma Expert's Insights

Pharmaceutical innovation moves slowly, often too slowly. Drug development takes over a decade, with costs exceeding $2.6 billion per approved therapy and a failure rate for new molecular entities above 90%. AI in pharma offers a way to rebuild this model. With the right data, structure, and algorithms, it enables higher-quality drugs, smarter trials, and faster decisions across every stage of development.
The global AI in pharma market is expected to grow from $4.35 billion in 2025 to $25.73 billion by 2030, reflecting the sector’s deep investment in scalable, intelligent technologies. This shift extends well beyond R&D, impacting trial operations, compliance, manufacturing, and commercial strategy.
from 25 countries outsourced software development to Relevant
We provide companies with senior tech talent and product development expertise to build world-class software.
This article, co-written with Maryna Todoriuk, MPharm, BBA, Trial & Client Senior Expert and oncology-focused clinical research leader, shows how AI software development services reshape the pharmaceutical industry. Together, we examine practical AI use cases in pharma value chain, grounded in real-world insights.
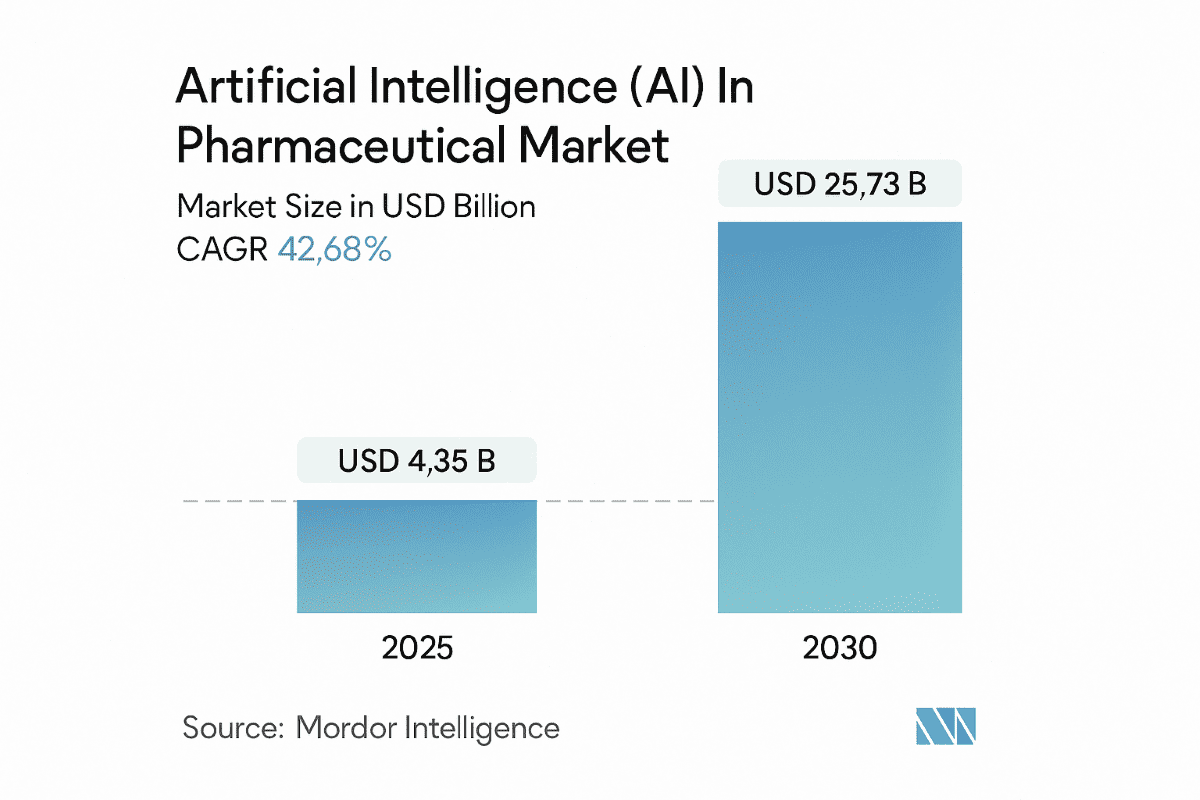
Source: Mordor Intelligence
The role of AI in pharma: Overview
The pharmaceutical industry now generates more data than ever before. Each new drug adds layers of lab results, trial protocols, patient records, and treatment outcomes. The challenge is no longer data collection. It is making sense of that data quickly enough to drive sound decisions. Artificial intelligence helps filter the noise, identify what matters, and guide teams with greater speed and clarity. It shifts the focus from reactive reporting to proactive insight.
Today, leading pharmaceutical companies treat AI as part of their core infrastructure. AI-first biotech firms such as Insilico Medicine and Exscientia use it to discover new drug targets. Global players, including AstraZeneca, Pfizer, and Sanofi, apply AI to regulatory compliance, pharmacovigilance, and trial simulations.
“In biotech and big pharma, artificial intelligence is a powerful tool that enhances and accelerates critical processes across the value chain,” says Maryna. “From early research to commercial deployment. By automating routine work and extracting deeper insights from complex datasets, AI frees professionals to focus on what matters most: strategic planning, scientific discovery, and improving patient outcomes.”
While earlier technologies focused on digitizing manual tasks, AI goes further. It selects high-potential compounds, flags operational risks before they escalate, and helps companies avoid low-return investments. For many organizations, progress now relies on faster and more accurate decisions enabled by AI.
Why AI is critical for the pharmaceutical sector
According to Maryna Todoriuk, AI supports one of the most essential goals in pharmaceutical development—answering the hardest questions early, before they turn into costly failures.
In clinical trials, this means identifying delays, spotting operational bottlenecks, and improving decisions before a single patient is enrolled. In drug discovery, AI narrows millions of potential compounds into focused, high-quality candidates. These efficiencies can reduce early-stage timelines by up to 40 percent and cut development costs by nearly 30%. By 2025, nearly one-third of new drugs may originate from AI-assisted discovery.
Maryna adds:
“AI also helps improve protocol quality during trial design, particularly in clinical operations. It can analyze complex assessment schedules, flag region-specific risks, and ensure feasibility across trial sites. Increasingly, AI is also used during the budgeting phase to forecast costs and reduce operational risk before the trial begins.”
These capabilities lead to more realistic, execution-ready protocols. Especially in high-risk fields such as oncology, predictive models help identify endpoints that are unlikely to succeed, forecast recruitment challenges, and highlight patients at risk of early withdrawal. AI does not replace expertise—it reinforces it with data-backed foresight that makes trial planning more precise and achievable.
Key technologies driving AI adoption in pharma
Based on Relevant Software’s experience, artificial intelligence in pharma does not rely on a single algorithm or tool. It brings together multiple technologies, each designed to support a specific phase of drug administration, development, approval, or delivery.
Machine learning
Machine learning models analyze vast amounts of biological data, clinical outcomes, and chemical structures to predict the performance of potential drugs. In early research, it quickly finds the most promising drug candidates. Later, it helps plan better trials by spotting risks such as patient dropouts or trial design weaknesses.
Advanced tools like deep learning give even more detailed predictions, such as how a drug works at the molecular level or which patients will benefit most. Many companies use AI outsourcing to bring these tools into their systems without disrupting compliance or research quality.
Natural language processing (NLP)
Each year, pharmaceutical teams face massive volumes of scientific literature, trial records, and safety data. Natural language processing (NLP) turns this unstructured information into clear, actionable insight. It filters key findings from studies, flags safety issues early, and prepares regulatory documents that match submission standards.
When teams include NLP in their AI implementation plan, they streamline drug safety tracking and speed up paperwork for approvals. It reduces errors and helps researchers spend less time on manual review and more time on meaningful analysis.
Computer vision
Computer vision enables scalable, image-based analytics across both R&D and manufacturing. During drug discovery, it analyzes cell images to detect changes under compound tests. In production, it inspects quality in real time, identifies problems with packaging, vials, or labels, and ensures full compliance with GMP (Good Manufacturing Practice) rules.
Within pharmaceutical QA operations, computer vision in healthcare outperforms traditional inspection methods, especially in tasks that require micro-defect detection or label verification at high volume. When integrated into modern pharmaceutical manufacturing software, these systems enable faster, more accurate quality control while reducing human error and production delays.
Generative AI
Generative AI in pharma redefines what’s possible in molecule design, trial simulation, and automation of documentation. These models use big data and computational power to mimic how the body works, design custom drug candidates, and test trial ideas without running real-life studies. This speeds up development and helps discover new treatments for diseases that currently have no cures.
AI in pharmaceutical industry doesn’t rely on a single model or technique. It draws strength from a combination of technologies, each addressing a specific stage in the pharmaceutical pipeline. Together, they reduce waste, accelerate insight, and make once-impossible decisions viable in real time. Relevant Software clients, including global pharma and biotech leaders, have already used this approach to streamline clinical operations, enhance data accuracy, and scale innovation across R&D and commercial teams.
Your next read: Generative AI in healthcare
Key use cases of AI in pharma
AI solutions for pharma are most beneficial when they are integrated into every step of the pharmaceutical process, rather than used in isolation, as this helps make processes faster, more accurate, and more informed. From early research through commercial rollout, here’s where AI in pharma and biotech drives real results.
Drug discovery and design
AI now replaces much of the early trial-and-error process that once defined drug discovery. Instead of manually checking thousands of compounds, researchers now use AI and machine learning to quickly analyze chemical structures and predict properties such as toxicity, the effectiveness of a drug, and the accuracy of its target. These tools help identify high-probability compounds and remove poor candidates early, which cuts both costs and development time.
According to Maryna’s experience, “AI empowers discovery teams by accelerating data analysis, uncovering hidden patterns, and identifying promising drug targets faster than ever before. It turns complex datasets into actionable insights, enabling smarter and more strategic decisions from the earliest stages of development”.
Generative AI in pharma adds another level of support. Large language models and task-specific neural networks now create new molecules tailored to exact targets. This approach is especially valuable where no obvious drug candidates exist. These systems simulate molecular interaction and suggest lab-ready synthesis routes.
Preclinical research
Once high-potential compounds are selected, teams move into the validation phase. At this point, AI continues to support decision-making by simulating how these compounds behave in biological systems. This allows researchers to estimate toxicity, absorption, and other risks before the compound enters the lab. The process reduces reliance on animal tests and supports faster, better decisions early in development.
Many pharma companies now structure their lab results in centralized healthcare data warehouses. This enhances the effectiveness of AI models by providing them with reliable, well-organized data to work with. Strong data pipelines help teams catch problems early and keep promising candidates on track.
Clinical trials optimization
AI helps improve trial outcomes by addressing common failure points: poor planning, unrealistic protocols, staffing issues, and financial risk. Predictive models forecast patient dropout, simulate site performance, and improve participant matching using historical and real-world data. These capabilities speed up recruitment, reduce protocol deviations, and enable real-time adjustments.
Clinical research expert Maryna Todoriuk notes that most trial failures stem from planning gaps. Common issues include underestimated budgets, missing access to essential services like comparator drugs, and poor vendor coordination. Geographic factors are often overlooked—differences in regulatory timelines, patient access, and local standards can delay startup and hurt recruitment. She emphasizes that real-world data should guide country selection and feasibility strategies, not assumptions.
Staff turnover at Contract Research Organizations (CROs) is another challenge. High turnover weakens execution, delays onboarding, and creates risk. Even well-managed trials can collapse if funding is withdrawn mid-study—a scenario Maryna has seen firsthand. These are not isolated problems but symptoms of planning without enough foresight.
“AI has the potential to reduce these risks by analyzing historical data on vendor performance, budget deviations, and site efficiency. Properly applied, it can help teams avoid repeat mistakes and build more resilient, execution-ready study plans. The trials that succeed tomorrow will be those planned with smart, data-driven systems today.”
Benefits of implementing AI in pharma
The pharmaceutical industry now moves beyond AI experimentation toward full integration. No longer limited to isolated use cases, AI in pharmaceutical industry informs strategic decisions across R&D, clinical development, and commercial operations. Below are four key benefits of AI in healthcare that have already demonstrated measurable value, both scientifically, operationally, and economically.
Reduced R&D costs and time to market
The cost and duration of drug development remain pharma’s most persistent challenges. AI addresses both. Machine learning models now cut early discovery time by up to 40% and reduce preclinical costs by 30%, based on multiple industry studies. In clinical trials, predictive models improve recruitment, refine trial design, and reduce risk in development cycles.
Many AI in pharma companies place predictive models earlier in the pipeline to avoid costly late-stage failures. These improvements result not from automation alone, but from using structured biological data and clean data sets that help teams fail earlier, set better priorities, and move with real confidence.
“A significant share of study budgets currently goes to Clinical Operations and monitoring activities,” says Maryna Todoriuk. “With automated data retrieval and AI-powered analytics, much of this manual work will be reduced. Integrated systems will increasingly handle patient data verification, so CRAs will no longer need to spend up to 75% of their time travelling to sites. This shift will reduce operational costs and ease the burden on both site teams and sponsors.”
Improved accuracy and decision-making
AI helps by finding patterns in scientific papers, safety reports, and trial results. Large language models assist in writing regulatory documents and extracting key insights, while computer vision analyzes lab images and medical tests with greater accuracy.
Relevant Software’s team of seasoned product developers and industry analysts reports that these AI technologies reduce manual errors and variability, especially in data-heavy environments. This means models are more accurate, important signals aren’t missed, and approvals happen faster based on real-world evidence.
Once operational accuracy improves, organizations can use AI to refine broader trial strategies and territory planning.
“When it comes to project and strategic management, properly trained AI models will also help significantly optimize trial planning and start-up,” Maryna thinks. “These models can analyze global territories to identify the most appropriate countries for specific clinical trials by considering key variables such as regulatory approval timelines, contract negotiation speed, and the availability of patient populations for the given indication.”
Better patient outcomes and experience
AI does more than improve healthcare operations — it drives better outcomes for patients. By analyzing real-world and genetic data, AI identifies patient groups more precisely and matches individuals with the right treatments, especially in cancer and rare diseases.
In clinical trials, AI tracks patient adherence, adapts interactions based on behavior, and keeps studies on schedule. After a drug is approved, AI supports pharmacovigilance by identifying safety signals early in real-world use. This allows manufacturers and regulators to intervene faster and improve patient care across broader populations.
This move from one-size-fits-all treatments to personalized care marks one of the greatest advances AI brings to the future of life sciences.
Competitive advantage through innovation
Pharma companies that use AI as part of their main systems move faster than those that run small, separate tests. These leaders rely on AI to explore various options, make informed decisions, and enhance their supply chain management.
This advantage now guides how pharma companies plan their technology. With tools like generative AI in pharma, smart systems, and data-driven strategies, AI-focused organizations stay ahead in both scientific breakthroughs and large-scale operations.
“That said, I do not believe the role of the CRA will be completely replaced by AI,” says Maryna. “On the contrary, human connection remains at the heart of clinical research. Building trustful, long-term relationships between investigators, sites, and sponsors is a cornerstone of successful trial execution. Therefore, the future belongs to CRAs who can skillfully combine technical proficiency in working with AI tools and digital systems, with strong interpersonal and communication skills to manage site relationships effectively.”
Key challenges of artificial intelligence in the pharmaceutical industry and their solutions
AI helps pharma move faster and support better decisions. But success depends on more than smart tools. It requires clean, trusted data, strong coordination across teams, and deep integration throughout the entire process.
To explore what separates AI success stories from stalled initiatives, we spoke with the VP of Delivery at Relevant Software. Based on their experience, here’s what pharmaceutical companies need to get right and how they can overcome the most common challenges.
Data privacy and security in healthcare
AI in pharma relies on data, but health information is some of the most private and sensitive out there. Every time that data is accessed, there’s a risk that it could be exposed. As AI tools become more complex, the likelihood of a data breach increases, especially when systems are used across different countries with strict privacy regulations. Many pharma leaders underestimate how costly it is to add privacy safeguards after launch.
“Privacy isn’t a checkbox,” says Anna Dziuba, VP of Delivery at Relevant Software. “It’s a strategic design principle. If security isn’t native to the system, the system doesn’t belong in healthcare.”
Many pharma leaders underestimate the cost of adding privacy protections after launch. That is why our healthcare software development teams embed safety measures such as data anonymization, file security, controlled access, and decentralized storage from the start. A system with built-in protections ensures AI remains safe and compliant. With proper oversight, AI serves as a valuable tool instead of a risk, even in fast-paced, global research environments.
Regulatory barriers and compliance issues
One of the most persistent disadvantages of AI in the pharmaceutical industry is explainability. Deep learning often lacks transparency, which conflicts with regulatory expectations. The U.S. Food and Drug Administration (FDA), the European Union’s AI Act, and agencies in Asia-Pacific, like Japan’s PMDA and Singapore’s HSA, require AI systems in healthcare to provide clear, auditable reasoning for decisions that affect patient safety and outcomes.
This creates tension for pharma teams. They want to adopt advanced automation and gain new insights, but not at the cost of compliance, approval delays, or audit failures. That’s why every AI project we deliver aligns with both internal quality assurance and regional regulatory frameworks, ensuring it’s not only effective but fully reviewable and ready for long-term use.
“You don’t sell regulators on AI,” says Anna. “You show them why your system deserves their trust. And that trust comes from clarity—both in outcomes and in how you got there.”
Integration with legacy systems
Many pharma teams still rely on disconnected, outdated systems that block real-time analysis and prevent the use of AI-powered tools. Even the best-trained models fail if companies do not integrate them into daily workflows, which wastes time and limits their impact.
Modernization does not require a full rebuild. With the right tools, such as APIs and cloud modules, companies can embed AI into existing systems without sacrificing compliance.
“We see AI initiatives fail not because the models are wrong, but because they never reach the people who need them,” notes Anna. Integration isn’t a back-end task. It’s the foundation of operational success.”
The future of AI in pharmaceutical industry
The future of pharma will use AI to create personalized treatments and run virtual trials. Let’s see some examples of how this is already happening.
AI-enhanced strategic planning
Pharmaceutical leaders increasingly rely on AI to support strategic planning. Intelligent systems forecast regulatory approval times, model contract negotiation cycles, and analyze population availability across global trial regions. This shift reduces guesswork and helps sponsors enter markets faster.
“When it comes to project and strategic management, properly trained AI models will also help significantly optimize trial planning and start-up,” notes Maryna Todoriuk. “These models can analyze global territories to identify the most appropriate countries for specific clinical trials by considering key variables such as regulatory approval timelines, contract negotiation speed, and the availability of patient populations for the given indication.
According to Maryna Todoriuk, centralizing planning data in a unified system enables faster and more efficient trial launches. This improves feasibility and shortens time-to-market for innovative therapies. She emphasizes that AI-driven strategic planning leads to better, faster, and more data-informed decisions, strengthening both speed and quality across clinical development.
Personalized medicine and precision drug development
AI is transforming drug development by shifting the focus from generalized populations to individual patients. It analyzes genetic and real-world data to identify the most effective treatment for each person. This improves outcomes, reduces trial risks, and helps avoid ineffective therapies.
“I would also emphasize the importance of applying a personalized and unique approach when developing an AI service. Each protocol, region, study population, and therapeutic pipeline presents unique challenges. Tailoring AI solutions to these specifics ensures higher accuracy, relevance, and value, which is exactly what this IT company delivers.”
Personalization is critical not only in treatment but also in how AI tools are built. In precision medicine, success depends on aligning technology with the unique context of each study and therapeutic strategy.
AI-powered digital twins for clinical simulations
Digital twins are AI-driven models that replicate patients, sites, or entire clinical trials. They allow researchers to simulate responses, test protocols, and identify risks before enrolling participants. This improves trial design, enhances safety, and reduces costly amendments.
Digital twins are especially valuable in complex studies with narrow inclusion criteria, such as oncology or rare diseases. They help optimize recruitment, dosing, and endpoint selection based on predicted outcomes.
In decentralized trials, digital twins simulate site performance, forecast dropouts, and support patient retention strategies. By enabling scenario testing in a virtual environment, they reduce uncertainty and improve execution across global trial operations.
The rise of explainable and ethical AI in pharma
As life science companies expand their use of AI, pressure rises to ensure it stays clear, fair, and easy to understand. Doctors, regulators, and patients demand trust in how AI reaches decisions. Explainable AI now holds greater importance, especially in drug development and the detection of safety issues.
In the years ahead, pharma companies will need to adopt governance frameworks, audit mechanisms, and clinical validation protocols to ensure their AI systems are not only powerful but also reliable, traceable, and fair.
“In summary, the clinical research landscape is evolving—but people will always remain its core. The most successful professionals in this new era will be those who embrace technology while continuing to lead with empathy, integrity, and purpose.” — Maryna Todoriuk, MPharm, BBA
Your next read: Future of AI in healthcare.
How we helped AstraZeneca cut costs and accelerate insights with AI
We began our cooperation through AI consulting services, as the client wanted to implement AI but had no clear plan for where or how to apply it. After a close examination of their operations, we discovered that AstraZeneca’s Medical Affairs teams faced major challenges with large volumes of unstructured data, including clinical documents and patient feedback. Manual review consumed valuable time and caused delays in delivering critical insights.
To solve this, our team developed an AI-powered solution on Google Cloud that organizes scattered data into structured, compliant reports that medical teams can actually use. We also built in security encryption and user access controls to meet strict compliance standards.
What changed?
- Insights arrive faster, and workflows operate with greater precision.
- Medical Science Liaisons now spend 40% less time on material preparation and focus more on strategy.
- The system processes over 35,000 medical records automatically, which cuts the manual workload significantly.
- Data accuracy rose by 25%, which drives better decisions across teams.
For pharma teams that manage high-volume, complex data, this project proves what becomes possible when AI addresses the right challenges with clarity, security, and speed.
Expert insight – Maryna Todoriuk, MPharm, BBA
“In summary, the clinical research landscape is evolving—but people will always remain its core. The most successful professionals in this new era will be those who embrace technology while continuing to lead with empathy, integrity, and purpose.”
Implement AI in your pharma business with Relevant
Building AI for pharmaceutical industry is not about installing a model or adding another dashboard. It is about fitting intelligence into real clinical operations, strict regulatory frameworks, and everyday workflows that already carry significant weight. If AI cannot adapt to those realities, it will not create real value, and too many systems fail exactly for that reason.
At Relevant Software, we build AI platforms that help pharmaceutical teams unlock what matters most: better use of the data they already have, earlier patient insights, faster identification of trial risks, and smarter field operations. Privacy, security, and compliance are not side projects. They form the base architecture, designed from the beginning to meet industry demands without compromise.
Our healthcare software development company has delivered tangible outcomes across the healthcare and pharmaceutical industries, helping companies move faster without compromising safety or quality. This experience has earned us recognition from Clutch as a Top AI Consulting Company, Top AI Deployment Company, Top Generative AI Company, and Top Computer Vision Company — but more importantly, it has earned us long-term partnerships with clients who trust what we build.

We start with a full review of your business, a map of your processes, and a clear definition of where AI adds real value. Many clients approach us with the idea of AI, but without a plan for its real purpose. Our role is to tie AI directly to your goals, your operations, and your long-term growth.
Have a vision in mind? Our AI experts are here to bring it to life. Contact us today.


Hand-selected developers to fit your needs at scale! Let’s build a first-class custom product together.


