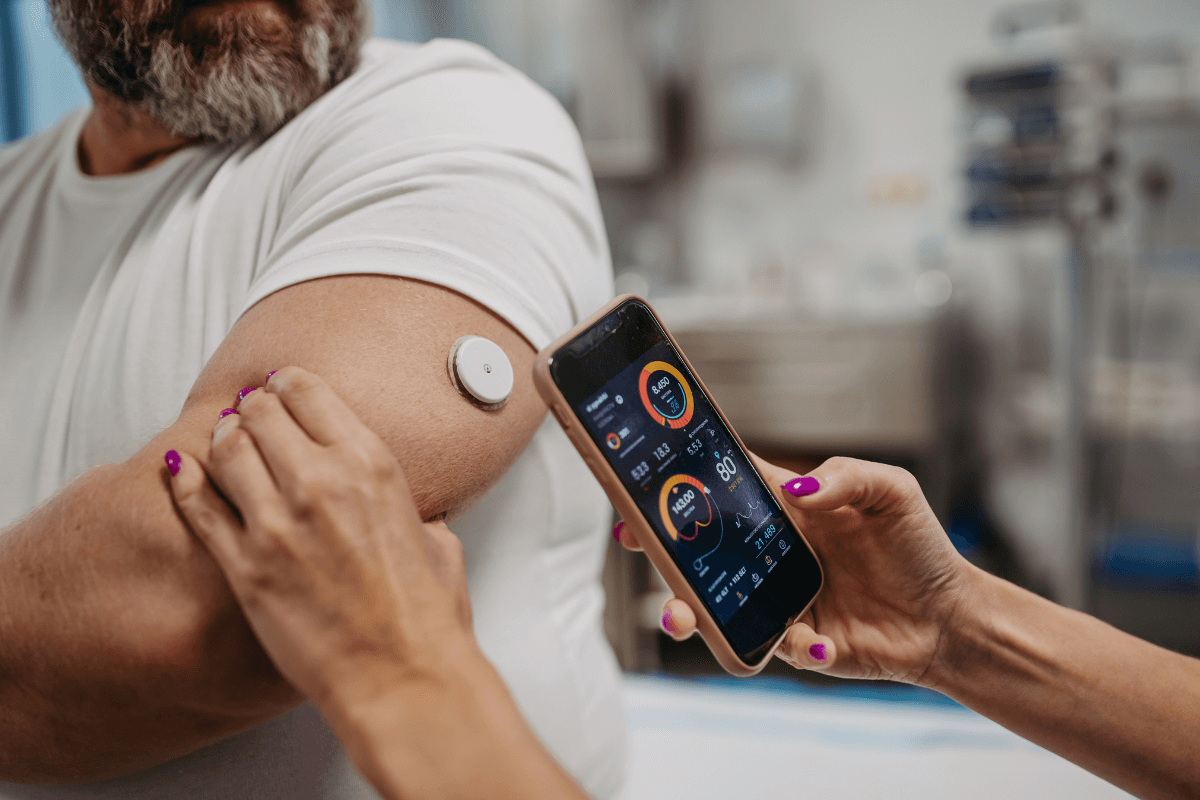
As new challenges emerge and patient needs grow, there are always workflows to optimize, systems to streamline, and costs to manage better. IoT software development services offer a viable solution to many inefficiencies and bottlenecks the medical sector faces today. Yet, when you rely on IoT medical devices to deliver critical patient care, security, and proper device function should come first. Although software and hardware are the key elements that require protection, IoT firmware development is equally crucial in ensuring the reliability and security of these devices.
Why do we need firmware for IoT devices at all, and why is it so important? IoT solutions for healthcare must operate flawlessly to provide accurate and timely patient care. Among the dozens of critical components that make up a successful IoT implementation, firmware in IoT is one of the most fundamental building blocks. Want to get this straight and know more about the role of firmware in device security and the peculiarities of firmware development? We shed some light on this often-neglected element of IoT success.
We provide companies with senior tech talent and product development expertise to build world-class software. Let's talk about how we can help you.
Contact usTable of Contents
Firmware is essentially the software that provides low-level control for a device’s hardware. It’s often referred to as “hard” software because it’s designed to be a permanent fixture within a device’s hardware solution. It resides in the system’s ROM (Read-Only Memory) or other permanent memory, where it holds the essential data and instructions that control the hardware’s functions. The “hard” aspect of firmware comes from its purpose: to provide long-term performance of medical devices with only minor updates or tweaks as necessary.
Then, what is a firmware in IoT in simple terms? Firmware in IoT is basically the device’s operating system that allows users to control the device according to their needs. It’s responsible for such functions:
The core distinction between software and firmware is their specific roles and functions within a device.
Firmware is embedded in the device and operates at a low level, controlling the hardware directly, whereas software runs on top of this, providing the features and functions users interact with. Firmware is rarely updated and tightly linked to the hardware to ensure smooth and secure device operation. In contrast, software can be easily updated or changed to add new features or fix bugs.
Here’s a quick table that outlines the key differences:
| Aspect | Firmware | Software |
| Purpose | Provides low-level control of hardware | Provides user-facing functionalities and applications |
| Level of Integration | Deeply integrated with hardware | Less tightly coupled with hardware, runs on top of firmware |
| Location | Stored in non-volatile memory (e.g., ROM, flash memory) | Stored in volatile memory (RAM) or on storage drives |
| Updatability | Rarely updated, updates are complex and risky | Frequently updated, easy to install new versions |
| Resource Management | Optimized for minimal resource usage | Can be resource-intensive, depending on the application |
| Security & Stability | Focuses on stability and security, often critical | Security varies, often prioritizes functionality and usability |
Firmware might not be the first thing you think of when picturing connected medical equipment, but it’s absolutely essential in the overall IoT architecture and for concrete devices in particular. IoT firmware development and embedded solutions are the main contributors to the optimal performance of IoT devices in healthcare.
In addition to being a connecting link between the device’s hardware and software, embedded firmware orchestrates device functions and enables smooth data transfer to backend servers. The overall IoT device security also largely depends on firmware. Consequently, any weaknesses in the medical device’s firmware can result in data compromisation, patient privacy violations, or even physical harm. To sum up, the key roles of firmware in IoT medical devices are:
Firmware for IoT devices consists of several essential elements that work together to enable device functionality and communication. What are they, and what role do they play?
The hardware abstraction layer, or shortly HAL, is an intermediary between the device’s hardware and the higher-level software that simplifies communication with the different hardware components. Without the HAL, software would need to understand the specific details of every hardware component it interacts with (sensors, actuators, etc.). In simple terms, the HAL is an interface that standardizes how software talks to hardware. Here’s why medical devices need HAL:
In practical terms, the HAL in a medical device might manage data from various sensors, control actuator functions, or facilitate communication with external systems. For example, in a smart insulin pump, the HAL would translate the software’s command to increase insulin dosage into specific electrical signals that operate the pump’s motor and metering system. This ensures that the pump delivers the correct amount of insulin without the software needing to understand the intricacies of the pump’s mechanical components.
Smooth communication between the connected devices is the essence of the Internet of Things technology. Here’s the list of communication protocols that enable data exchange in IoT systems:
Medical devices are lucrative targets for hackers since they transmit sensitive health information and are easier to compromise due to their often limited security measures. Therefore, encryption, authentication, and authorization protocols are a must for proper patient data protection.
All medical devices are equipped with sensors – elements that actually collect information from the surrounding environment or from the patient’s body. Yet, one sensor can also be a standalone solution that functions independently and transmits data. Anyway, we can distinguish several types of sensors:
For the whole IoT system to read and process data, the sensors they have should integrate with firmware. A few techniques make this possible.
A large portion of sensors generate analog signals, which need to be converted into digital data that the device’s microcontroller can understand. For this purpose, analog-to-digital conversion is applied. Then, firmware in IoT uses calibration techniques to fine-tune the sensor readings to ensure they are as precise and reliable as possible. So, if we take an ECG monitor, the firmware processes the electrical signals from the heart, filters out noise, and translates them into clear, readable data for healthcare providers.
When we talk about life-saving equipment like pacemakers or insulin pumps, power management is a critical concern. These devices usually operate remotely and rely solely on battery power efficiency. A sudden power down of such a device can cause catastrophic consequences and lead to serious injury or even death for the patient.
The best solution for ensuring a reliable and uninterrupted power supply is good IoT device management, which helps monitor device performance, power levels, and other critical aspects remotely. With it, healthcare providers can:
The firmware development process needed for an IoT medical device is not very much different from creating software. Certainly, it involves some unique challenges and careful considerations, especially when it comes to safety and compliance. So, there’s no room for error. Nevertheless, IoT firmware development shares the essential steps of developing software or a mobile app, which we outlined below.
It all starts with understanding the specific requirements of the medical device. If you hire IoT developers, you need to gather the IoT firmware development team members and all stakeholders to define and outline such requirements:
It’s also vital to emphasize the importance of compliance with regulatory standards at this stage. Medical devices, including all their hard and soft components, must follow strict regulations, such as those set by the FDA in the US or the European Medicines Agency in the EU. The purpose of FDA medical device regulations is to ensure that devices are secure and safe to use. Therefore, custom firmware development should take it into account from the start since it will tell which data security protocols to implement, what level of testing is required, and even the choice of hardware components.
FDA classification of medical devices
| Class | Risk | Controls | Submission |
| I | Lowest | General | Exempt510(k) |
| II | Moderate | General and special (if available) | 510(k)Exempt |
| III | Highest | General and PMA (pre-market approval | PMA |
Once you have a clear vision of medical device specifications and the scope of work, you can get started with designing the architecture. It will describe the structure, define how components interact, and establish the communication protocols and data flow within the system. A well-thought-out firmware architecture determines the system’s scalability, maintainability, and capability to handle the complex tasks required in a healthcare facility.
The next step in IoT firmware development is the selection of the right development tools and programming languages. They should be modern, powerful, and, most importantly, compatible with the chosen hardware components of the medical device. Although C++, C, and Assembly are some of the most popular options for low-level programming, the choice depends on the complexity of the device, the desired level of control, and the team’s firmware development expertise. The point is the selected tools should support the IoT firmware development process, facilitate debugging, and enable efficient code writing.
Once you establish the development environment, you can get to work on the main embedded firmware development process. No one will argue that generally good coding practices are essential, especially in the constrained world of embedded systems. Clean, secure, and well-structured source code is the foundation for reliable firmware.
To achieve that, your IoT firmware development team should write binary code that smartly uses the limited memory and processing power of IoT devices. What’s more, they should implement strong error handling so the system is capable of dealing with unexpected situations. Also, modular design will help a firmware developer improve code reusability and simplify testing and maintenance.
There is no need to say that bugs and vulnerabilities in IoT medical equipment can have serious consequences. Therefore, firmware testing should be careful and exhaustive and comprise all types of tests:
Developers should also employ debuggers, simulators, and code analysis tools to catch glitches and fix them as early in the IoT firmware development stage as possible.
Finally, it’s critical to test the firmware regarding compliance with medical device regulations. The custom firmware development company you cooperate with must make sure it meets the required safety, performance, and security standards. Meeting these regulations often requires a lengthy and detailed validation process, which usually includes creating comprehensive documentation about the development and testing procedures and going through third-party audits.
Every healthcare organization that decides to invest in embedded firmware development may face several challenges, which are largely due to the specific nature of IoT systems and user requirements. Let’s look at the most common problems encountered when developing firmware.
The embedded firmware development for healthcare applications demands special attention to regulatory compliance with global standards and different laws of the countries they are intended to serve. This means that in addition to universal safety and quality standards like ISO (for quality management systems for medical devices) and IEC, the firmware must also comply with regulatory bodies like the FDA (Food and Drug Association) in the United States and the European Medicines Agency (EMA) in the EU.
This may not be the easiest task, and it definitely requires relevant expertise. Building a fully compliant solution involves a deep knowledge of the specific requirements each regulatory body imposes concerning clinical data standards and cybersecurity measures. The process demands scrupulous documentation and validation to prove that the firmware meets all safety, efficacy, and quality criteria.
Ignoring these regulations is a showstopper. Non-compliance can cause delays in device approval, incur hefty fines, or even prevent you from bringing the product to market at all. In the worst-case scenario, a device that doesn’t meet standards can be recalled, which will tarnish a company’s reputation and result in great financial losses.
When your pacemaker or insulin pump is connected to the Internet, the data it transmits and receives is at the highest risk without strong protection. It isn’t a question of username or password data; we talk about personal health data that, if compromised, can have severe consequences for patients: identity theft, financial loss, and up to physical harm. Unfortunately, unauthorized access, data breaches, and malware attacks are all too real. Around 82% of healthcare institutions have fallen victim to cyberattacks that targeted their IoT medical devices.
To mitigate these risks, consider the following strategies, which are best to employ yet during the IoT firmware development process:
Firmware in IoT should be reliable and perform well for the medical device to function properly. Given that connected medical equipment might need to operate in critical environments like emergency rooms or intensive care units, a malfunction could mean the difference between life and death. In such high-stakes situations, even a minor error or delay in the device’s performance can be critical, which makes it clear the dependability of the firmware is absolutely crucial.
Well, the strong foundation of firmware is laid down during the design and IoT firmware development processes and calibrated during the testing phase. Yet, there are also some effective performance optimization techniques that will let you set up the device for peak performance in the long run. Memory management is the first thing to consider, as it ensures that the device can handle its workload without running out of resources or crashing. Meanwhile, code optimization for speed that may involve removing unnecessary functions and clearness will enhance device responsiveness.
Although firmware is typically built to be stable and unchanged, keeping it secure demands periodic updates. And it’s vital to keep it up to date with the latest version. So, why are firmware updates on IoT devices necessary? First, timely IoT firmware updates address the device’s existing security vulnerabilities and fix bugs that impact device performance or user experience. Secondly, they introduce new features that improve device functionality. Finally, firmware updates help you maintain compliance with healthcare regulations.
How can one establish a good IoT firmware update architecture? There are several methods to deliver updates securely:
The biggest challenge of firmware updates for medical equipment is possible disruption. Unlike consumer gadgets, medical devices are often in continuous use and can’t be taken offline without thorough planning. To avoid any negative impact on patient care, updates must be carefully scheduled and tested beforehand so they don’t interfere with the device’s operation. In this case, having redundant systems will allow one system to take over while the other is being updated.
Compatibility and reliability are other concerning issues healthcare practices encounter. An update must work seamlessly with the existing hardware and software without causing conflicts or degradation in performance. Therefore, you should conduct a number of tests to verify that the update does not introduce new issues. Furthermore, you should have a contingency plan for rolling back updates if something goes wrong to be able to quickly revert the device to a stable state.
It’s possible to speak a lot about the benefits and importance of software and IoT firmware development, but we believe it’s better to demonstrate. Here are a couple of our clients who we helped with IoT product development and came out of it.
Sensor Innovation, a company offering a modern system for monitoring industrial and residential sites, turned to Relevant to improve the functionality and user interface of its IoT product.
Our team has completely revamped the platform’s UI, which considerably simplified sensor management (their weak point). We designed an easy-to-use, intuitive IoT dashboard where users can effortlessly manage user accounts, view info about buildings, and monitor devices. A customizable building map feature that we added lets end users visualize sensor locations and see their statuses. Additional functionalities we built to provide an overview of humidity, temperature, and other critical environmental factors and create reports.
The new version of the platform is more intuitive and efficient. The weather timeline feature minimized false alarms due to weather data integration, while a building map optimized sensor coverage.
Airthings, a manufacturer of indoor air quality monitoring products, partnered with Relevant to develop a dashboard that will visualize air quality readings from their growing network of devices worldwide. Airthings’ existing system required a serious upgrade to effectively manage that data and allow the company to monitor their devices located globally. The challenge was to ensure the data was collected, processed, and displayed without lag, even if you needed the information from numerous devices at once.
What we did:
Our solution helped Airthings attract more users and effortlessly monitor device distribution across the globe as well as identify potential new markets.
Firmware in IoT is the foundation of a powerful IoT ecosystem healthcare organizations can rely on. As the number of connected medical devices continues to grow, the importance of IoT firmware development will only increase. Firmware development, along with its management, is no easy feat, as it covers everything from maintaining strong security to handling complex business logic. That’s why healthcare IT managers and practitioners turn to IoT development companies for help.
At Relevant, we have strong expertise in all questions related to IoT development, deployment, and maintenance, as well as experience collaborating with healthcare companies. So, we can safely say that we are well-informed about the industry’s complexities. Key areas of expertise we offer:
If you want to strengthen your IoT strategy or learn more about adopting IoT technology, use the ‘Contact us‘ form to discuss your options.
What if your bank could complete in minutes what currently requires hours, without adding a…
Forecasting demand has never been more complex—or more critical. Traditional models, constrained by rigid statistical…
Did you know that hospitals generate around 50 petabytes of data each year, including clinical…